The doc discusses the qualification system for your tablet compression machine. It describes the techniques of design and style qualification, set up qualification, operational qualification, and functionality qualification. Layout qualification establishes that the machine design satisfies prerequisites.It's also possible to herald external consul
New Step by Step Map For parts of prescription
I've acquired a great deal of with the medical doctor and Alloy and also the YouTube channel! Countless excellent capable Women of all ages standing up and stating no more it's not Okay and we’re gonna repair this now. I am so thrilled to become Component of Alloy! I have referred every girl I'm sure to the location.”Most effective Existence wi
Top latest Five IPA 70% solution Urban news
IPA kills micro organism by detrimental the mobile wall of an organism. Drinking water plays a vital part in catalyzing this response and denatures the proteins of vegetative mobile membranes — equally h2o and alcohol get the job done in to the microorganism, producing its walls to burst and dissolve speedily.Diluted disinfectant solution, that's
Detailed Notes on sterility test failure investigation
The investigation process must describe what info ought to be documented: The key reason why for your investigation, including what happened, when, and where by; initial evaluation such as checklists; the laboratory supervisor’s evaluation; aspects with the investigation system; and executed useful investigation, retests, and conclusion on the in
Not known Details About importance of cgmp in pharmaceutical industry
Batch manufacturing and Regulate data shall be organized for every batch of drug product produced and shall include things like total information and facts relating to the production and control of each batch. These documents shall include:Nissin Foods, a renowned title in the food items industry, confronted delays as a result of its reliance on pa
 Charlie Korsmo Then & Now!
Charlie Korsmo Then & Now! Jeremy Miller Then & Now!
Jeremy Miller Then & Now!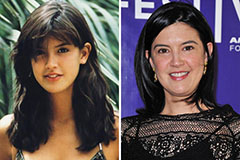 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Andrew McCarthy Then & Now!
Andrew McCarthy Then & Now! Erika Eleniak Then & Now!
Erika Eleniak Then & Now!